
[6 min read]
In this article:
- CAR T therapy, a type of immunotherapy, is used to treat certain types of cancer, with researchers working to develop it as a treatment of other disease, including lupus and multiple sclerosis.
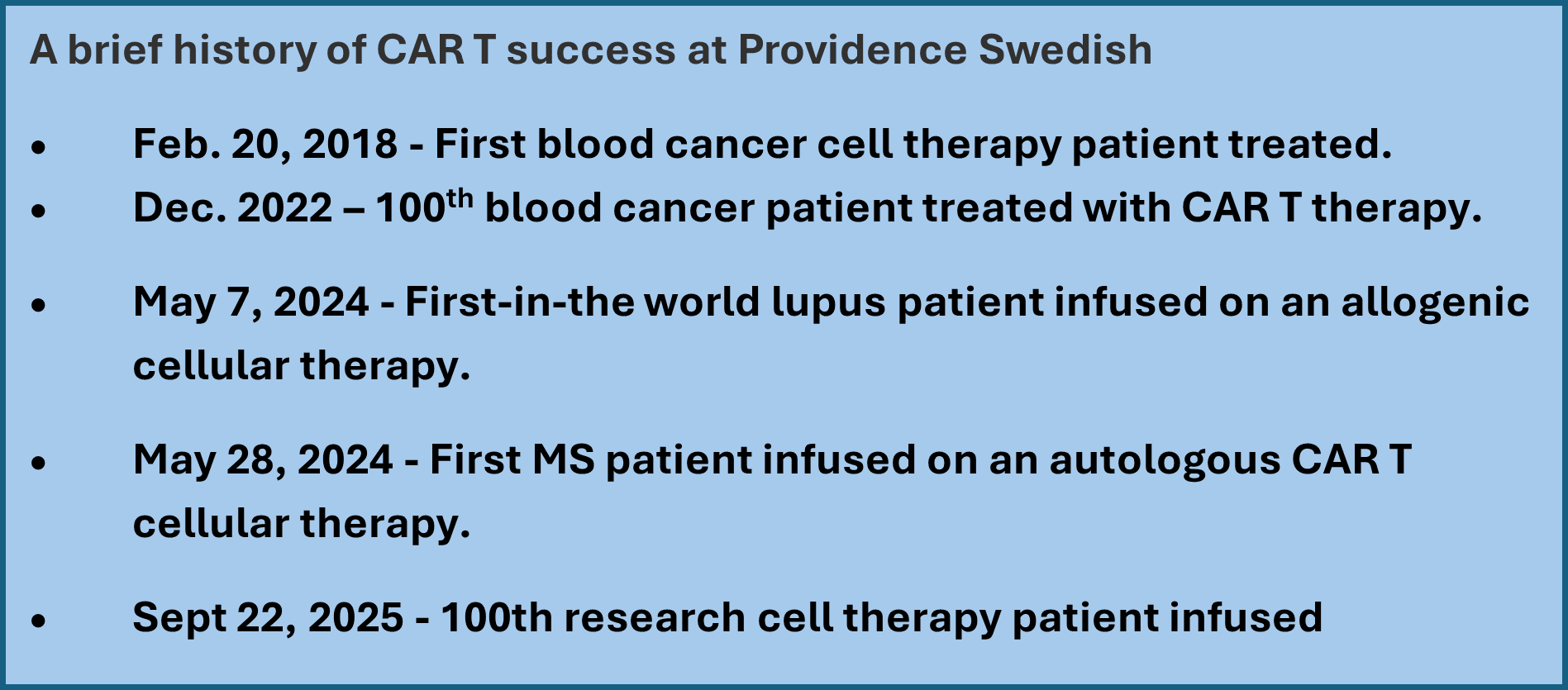
- On Sept. 22, a 100th Providence Swedish patient received CAR T therapy treatment through one of 24 research protocols, which will be developed into larger clinical trials studying the therapy for a host of conditions and diseases.
- Providence Swedish is the few centers in the world where many of these treatment options are currently available.
In late September, Providence Swedish achieved a significant milestone: our 100th unique patient treated through advanced research around chimeric antigen receptor T cell (CAR T cell) therapy. This extraordinary number and the partnerships and level of skill behind it, represents the expansion of once-unimaginable treatment pathways for patients with cancers such as lymphoma, leukemia and multiple myeloma, and autoimmune diseases, including multiple sclerosis (MS) and lupus. (In photo above, Providence Swedish researchers and caregivers during the first-ever allogenic, or off-the-shelf, cell therapy used to treat an autoimmune disease. In photo below, researchers prepare a cell therapy infusion.)
On Sept. 22, a 100th Providence Swedish patient received cell therapy treatment through one of 24 research protocols— think of them as plans or instruction manuals —which will be developed into larger clinical trials studying CAR T therapy for a host of conditions and diseases. Providence Swedish is one of just two sites in Washington state performing this level of CAR T therapy investigation.
“It’s certainly very exciting,” says Tenzin Tsomo, manager of clinical research for Hematology and Cell Therapy at Providence Swedish Cancer Institute. “When you look at the whole timeline CAR T is fairly new compared to a lot of the treatments we use in oncology and for other patients with other diseases. The first patient ever treated [with CAR T] was in 2010, so it has not been a very long timeline until 2025.”
“With these protocols, not all, not [even] many of these treatment options are available to everybody across the United States, or the world. Companies [that we partner with for research] select few institutions to run these phase-one trials just because they are safety trials, so they generally choose less than 10 sites across the U.S., or the world if it's a global study. And then we get to make these treatment options available for our patients. So, 24 protocols in phase one in collaboration with the sponsors is an immense achievement for Swedish and research here at the Providence Swedish Cancer Institute.”
CAR T cell therapy in oncology
CAR T cell therapy is a form of immunotherapy that uses genetically engineered T cells—a type of white blood cell—to destroy cancer cells by recognizing an antigen on their surface. Swedish research teams began using CAR T therapy in 2018 clinical trials, and by late December of 2022, they had completed their 100th U.S. Food and Drug Administration-approved treatment for blood cancer.
There are two types of CAR T cells used in therapy: autologous, in which a patient’s own cells are taken, modified and infused to fight their specific cancer, and allogenic, in which multiple patients are treated using the same CAR T cells. Allogenic transplants are particularly important because many oncology patients can’t wait the 28 days it takes to develop autologous cells. A significant finding related to the current research is that manufacturers have been able to cut development of this personalized therapy in half to 14 days, which is a major benefit to patients and their families.
CAR T cell therapy for MS and Lupus
According to the National Multiple Sclerosis Society, approximately 2.8 million people worldwide are living with MS, an unpredictable and often disabling disease of the central nervous system. Lupus, a chronic autoimmune disease that can affect various parts of the body, impacts around 5 million people globally, according to the Lupus Foundation of America.
For MS and Lupus, CAR T cell therapy could fundamentally alter how we approach treatment by reprogramming T cells to moderate immune responses and promote self-tolerance. While research is still in relative infancy, CAR T therapy for autoimmune conditions represents a significant shift, offering hope beyond the limits of conventional therapies. This past May, Providence Swedish was the first-in-the-world site to treat a Lupus patient with allogenic CAR T cell therapy.
Teamwork builds the path forward
Jennifer Hansberry, executive director of Research at Providence Swedish, emphasizes that this work requires thoughtful, committed partnership at every level to succeed, and ultimately, to best serve patients.
“There is an incredible amount of care coordination between research teams, clinical teams and resources to ensure that we have the right safeguards and locations and the right people on every team,” says Hansberry. “This means we have the best people and the best preparations defined by the protocol. It’s not like we can wait a day if someone is out of town. There is an extraordinary amount of work that goes into mapping out every step months in advance. That’s why being able to do 100 is phenomenal.”
This fusion of cutting-edge science with compassionate patient care underpins every aspect of research at Providence Swedish, affirms Neil Bailey, director of Oncology Clinical Research at the Providence Swedish Cancer Institute.
“Alongside the complexity and coordination is the skill that our physicians and teams have to do these trials,” says Bailey. “There are only 150 to 200 sites that can do what we are set up to do. We started this over 10 years ago — and here we are.”
Learn more and find a physician or advanced practice clinician (APC)
If you or a loved one have questions about cancer diagnosis, treatment or care, the experts at the Providence Swedish Cancer Institute are here for you. We can accommodate both in-person and virtual visits. To talk to someone or make an appointment, call 1-855-XCANCER.
You can also learn more about available clinical trials. Our physician investigators and researchers are involved in hundreds of ongoing trials involving most types of cancer.
Whether you require an in-person visit or want to consult a doctor virtually, you have options. Swedish Virtual Care connects you face-to-face with a nurse practitioner who can review your symptoms, provide instruction and follow up as needed. If you need to find a doctor, you can use our provider directory.
Additional resources
Providence Swedish is transforming cancer care and research
Research at Swedish is saving lives. Your support will help it continue.
Harnessing the power of the body’s own immune system to treat (and defeat) cancer
Emerging hope for patients with small cell lung cancer
This information is not intended as a substitute for professional medical care. Always follow your health care professional's instructions.





















